IN SEARCH OF HIV
Analysis of the value of the tests used for 'HIV infection'By Fabio Franchi
Leadership Medica Sept. 1998
"The task of researchers is to try to falsify as many theories as possible."
-- Karl Popper"Is there a danger, in molecular biology, that the accumulation of data will get so far ahead of its assimilation into a conceptual framework that the data will eventually prove an encumbrance? Part of the trouble is that excitement of the chase leaves little time for reflection. And there are grants for producing data, but hardly any for standing back in contemplation".
-- John Maddox (1)
AbstractThe diagnosis of HIV infection is made according to the results of particular tests, of which the most important is the Western Blot, also named “confirmatory test”. This one, on its turn, may or may not be corroborated by other tests. But, a methodological and comparative analysis of anyone of them let one conclude for a complete lack of specificity (and real validity). Antibody tests, viral culture, direct detection of the virus, electronic micrographs of HIV, HIV isolation allow one to find foundamental errors in certain premises and in some essential logical constructions.
The argument that what is not proved by single tests can be proved by them together is not valid from a scientific point of view. The paradoxical result of this critic examination of the matter is that anyone of the phenomena attributed to the virus is explainable without the need to postulate its existence; Electronic micrographs of what has been considered “pure HIV” - and only recently published - seem to confirm this thesis completely.According to the AIDS infectious theory, the HIV infection is active since from the first phases and during the years and it is very difficult for the organism to get rid of it. Proper tests reveal its presence. Those which at the moment are at our disposal are essential not only for the diagnosis (2), but they are used as indicative elements with prognostic and therapeutical purposes (3).
As science for definition is open to every control and all its demonstrations must be proved on the basis of logical rules, this study has the object of verifying if there is effective relation among the different “AIDS tests” and the meaning attributed to them. We shall examine the most important: the tests of the antibodies (the ELISA, the Western Blot and the p24), the viral culture, the genetic identification with amplification methods, the viral isolation, the micrographs of HIV. We shall analyse and criticize the procedure followed at present, as presented in the updated specialized texts including “AIDS, Biology, Diagnosis, Treatment and Prevention” (2) published in 1997, edited by several top researchers including A. Fauci, former manager of the National Institute of Health.
In other words we tried to answer to the crucial question as the great clinician and science methodology scholar, prof. Augusto Murri would have formulated it: “Why have I to believe that the AIDS test meaning is equivalent to an infection with the HIV virus?” He thought it was essential, during one's life, to be in the habit of criticizing before of accepting information.
Introduction
According to the AIDS infectious theory, the HIV infection is active since from the first phases and during the years and it is very difficult for the organism to get rid of it. Proper tests reveal its presence. Those which at the moment are at our disposal are essential not only for the diagnosis (2), but they are used as indicative elements with prognostic and therapeutical purposes (3).
As science for definition is open to every control and all its demonstrations must be proved on the basis of logical rules, this study has the object of verifying if there is effective relation among the different “AIDS tests” and the meaning attributed to them. We shall examine the most important: the tests of the antibodies (the ELISA, the Western Blot and the p24), the viral culture, the genetic identification with amplification methods, the viral isolation, the micrographs of HIV. We shall analyse and criticize the procedure followed at present, as presented in the updated specialized texts including “AIDS, Biology, Diagnosis, Treatment and Prevention” (2) published in 1997, edited by several top researchers including A. Fauci, former manager of the National Institute of Health.
In other words we tried to answer to the crucial question as the great clinician and science methodology scholar, prof. Augusto Murri would have formulated it: “Why have I to believe that the AIDS test meaning is equivalent to an infection with the HIV virus?” He thought it was essential, during one's life, to be in the habit of criticizing before of accepting information.
ANALYSIS OF THE ANTIBODY TESTS
The tests of the antibodies we shall examine are the Elisa, the Western Blot, the p24 antigen capture assay.
ELISA
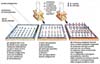
Fig. 1 - ELISA test procedure scheme.
In Italy, the screening test (ELISA), “provided with a good sensibility (near 99%) and a lower specificity, valued about 90-95% in 1988”(4), is used at first to identify the HIV infection. However these percentages must not deceive, in fact the test predictive value changes depending on the infection prevalence in a certain population. According to a theory expressed in 1988 by the AIDS National Commission (4), in case of screening of the Italian prison population, in comparison with 6,902 real positive individuals there should have been 1.751 false positive; on the contrary, in case of blood donors, in comparison with 198 real positive, a good 999,990 should have been false positive (505 times more!). In the over mentioned text (2) the authors assert that the progressive improvements took sensibility of the test to 95% and its specificity to 99.8%, in high-risk population. Nevertheless they admit that “the positive predictive value can greatly fluctuate depending on the population they study”. Actually this happened. Depending on the information published on the English authoritative medical magazine The Lancet (5), in 1990 on 20.2 million ELISA tests made in Russia, 20,000 were positive, but only 112 were confirmed by the WB; in 1991, on 30 million ELISA tests, a good 30,000 were positive, but of these only 66 were confirmed by the Western Blot, that is a minimum percentage (0.002%).
“Then as the ELISA test has been planned to optimize the sensibility to the detriment of the specificity, it should not be used by itself for the HIV-1 infection diagnosis without a confirmation test (2), although in some Countries is used alone.
Western Blot
Fig. 2 - Western Blot test procedure scheme.
The Western Blot is commonly used as a confirmation test of HIV infection; the combination of the Elisa and the WB should have a “positive predictive value higher than 99% for low-risk and high-risk population” (2).
However even the WB does not seem to be so specific as sustained if we analyze some data at our disposal.
A) Even official sources acknowledge that “for reasonable disagreements it was very difficult to code the definition on what a positive Western Blot should be considered” (2) . Since 1993, in United States there were just 5 official criteria, and only one (indicated in Du Pont's diagnostic kit) was approved by FDA (Food and Drug Administration). This was the most restrictive one and was used by a few laboratories. If only this criterion had been used , in United States only the 50% seropositive individuals would have been confirmed as really infected (6)! In Italy in a publication of the Health Ministry directed towards G.P. (7), the authors denied there had ever been such a problem. On this subject they affirmed: “in most cases, quite apart from the criterion used, the test results correctly coded when the individual is infect”.
The remedy to such a situation was found simply standardizing the different criteria without reconsidering the past and without explaining the scientific reasons for which they opted in that way.
Afterwards the CDC identified their criteria with the ASTPHLD's ones and in 1993 the United States Red Cross conformed adopting the ASTPHLD/CDC's advice”(2). “According to the American authority ASTPHLD, a positive result must present at least two of the three main bands of diagnostic meaning (antigp-41, anti-gp120/160, anti-p24)”.
B) The reading of the WB is visual and therefore is liable to interpretation from the operator, a variability factor not negligible. “Its technique (note: the Western Blot) was not standardized, the importance and the consequences occurred in the laboratories have not been yet measured.
Their results must be interpreted; the criteria for these interpretations vary not only from laboratory to laboratory, but even from month to month..” (8). The Consortium for Retroviral Serology Standardization (CRSS) made a quality control study*and sent 19 aliquots of the same serum to 19 different USA reference laboratories. Results comparison has shown an incredible differences of the number of bands and of their intensity (6).
Fig. 3 - WB results from the same serum and obtained
by 19 different laboratoires are shown.C) The antigenic preparations are not purified.
“The presence of bands in positions not corresponding to well-known virus antigens is a rather common finding and we suppose it is the consequence of contaminants in the preparation...” “The molecular weight of these aberrant bands may vary either from company to company or even from lot to lot of the same producing company” (2).
Here is Zolla-Pazner's comment in 1989: “A confusion on the identification of these bands (note: the results of the Western Blot test ) resulted in incorrect conclusions in experimental studies. [...] A re-interpretation of the already published results could be necessary”(9). Re-interpretation that has never been made.
D) The bands considered “specific” in many cases does not indicate an HIV infection. Some examples follow.
- Even if the more restricting criteria were employed, Lundberg noticed that 10% control sera, which included blood samples from blood banks, had a positive WB (6).
- A study(10) shows that from 2 to 49% tested patients can have a reaction to the WB (unspecified WB), more frequently caused by cross-reacting antibodies.
- A simple vaccination against flu can give a positive result too (11).
- Transfusion of own irradiated blood induces the formation of the same antibodies (12).
- Among the homosexuals and bisexuals who resulted negative for ELISA and PCR, 20%-30% can have either one or more bands present in the Western Blot.(2)
- There are even more amazing data concerning dogs and published in 1990. Writing on Cancer Research, Strandstrom at al. reported that 72/144 (50%) dog blood samples obtained by the Veterinary Hospital of the University, California, and tested with the Western Blot reacted with one or more HIV recombinant proteins [gp120--21.5%, gp41--23%, p31--22%.p24--43%]”(13). As the Authors think that dogs can not be infected with HIV, one must conclude that these data are a further prove of the frequent antibody cross-reaction with many proteins different from HIV ones”.
Remarks: The fact that the bands are considered unspecific if they are found separately, but virus-specific if they are present in a number of two or more, seems to refer not to considerations of a biological kind, but to a convention, a legal agreement changeable in time and space (in fact it is different from nation to nation). According to a convention accepted since a few time ago by many authors, three bands in a clinically healthy blood donor's WB were considered false positive reactions. According to the same convention, three bands of the same blood donor, this time presented to an Insurance Company for a life policy, should be considered true positive.
The researchers group from Perth who analyzed in depth the WB meaning, concluded that the proteins considered viral proteins are not peculiar to HIV, but one cannot distinguish them form proteins of a cellular source. In particular, according to the review by Eleopulos et al.(14) which has not yet been refuted up to now, the gp41 should correspond with actine; the p18 and p24/25 with the two myosin sub-units (p24 is the unspecific band found more frequently in “uninfected” individuals); the p32 should be identical to the beta chain of the antigen DR of the histocompatibility class II; the gp120-160 should not be other than gp41 oligomers.
The p24 antigen
In U.S.A. the evaluation of the p24 antigen capture test was included among the assays which are carried out to each blood unit (and it is considered as an “HIV direct evidence”). Fauci et al.(2) think that: a) the p24 corresponds to virus capsid antigen (core) which is highlighted by a serum containing the related monoclonal antibodies (anti-p24) associated to a detector system; b) however the test is not useful like a primary screening mean in establishing a precocious HIV infection diagnosis; c) the p24 level increasing corresponds presumably to the blast in virus replication, which can be observed by other methods like plasma viraemia; d) the p24 level usually falls under the determination threshold with conventional methods and like the antibody anti-p24 starts developing during seroconvertion and can remain in that state during the following asymptomatic infection years. Only 20-30% of the individuals with asymptomatic infection has detectable levels of p24 serum.
However there are many perplexities on its real meaning. Quantity data indicate that 50 pg and sometimes more of core protein (p24) may be found in seropositive patients. If a retrovirus weight is of 10-3 pg and half of its weight is constituted by p24 (15), then this quantity corresponds to about 100,000 viral particles, according to other authors and on other premises even to 500,000 particles (16)! Then if all this protein came from virions, these patients should be highly viraemic (17, 18). In other words there should be a direct correspondence among the p24 levels, “virus isolation”, “viral RNA” levels (said also “viral load”). On the contrary it is not so. Results are irremediably different from the ones one should expect. For example a paper reports the presence of antigenaemia, but that no virus could be isolated by 31 antigenaemic patients, even after an extended culture (19).
Other studies report similar remarks. We shall mention some of them:
1) In half of the cases when a person had a positive test, afterwards he had a negative one without making any treatment which could alter the p24 levels... the test is clinically erroneous and should be interpreted with much caution”.(20)
2) The p24 antigen is found neither in all seropositive subjects nor in individuals with clear AIDS. In a study , the PCR (Polymerase Chain reaction) and p24 were used to detect HIV in patients in in various stages, from asynptomatical to AIDS individuals. They found the p24 in 24% and HIV-RNA in 50% of patients (21).
3) More than 29% of the patients who receive a transfusion from seronegative individuals, become positive for the anti-p24 antibody (22), even “on infection absence”.
4) The unspecificity of the p24 antigen assay is so obvious to be accepted by an authority recognized in the field, Philip Mortimer.et al- from Public Health Laboratory Service in England.” “Experience proved that neither virus culture, nor p24 tests are of great value in diagnostic tests. They can be either insensible or unspecific” (23).
Conclusion
The antibody tests (the ELISA, Western Blot and p24 antigen capture assay) for the many reasons over explained, are non able to indicate with reasonable assurance they can face an HIV infection and so the assertion that WB positive predictive value (24) can be considered higher than 99% does not correspond with reality. These tests show reactions of a variable or rather undetermined specificity, so that they cannot allow a distinction between cross-reaction and “HIV infection”.
THE DIRECT IDENTIFICATION OF THE VIRUS
It is a common conviction that the antibody tests should be ratified and in a certain way verified from other ones which are able to highlight the virus itself, tests able to identify it directly. However, this direct determination is such only apparently. It can only record some phenomena which are considered associated to the HIV in an unequivocal way. Virus direct identification is divided into 2 main categories (2): the virus culture and the direct identification of the viral nucleic acids (Southern Blot, amplification techniques like the PCR,TR PCR, bDNA, NASBA).
The viral culture
Usually virus isolation involves a patient's cell co-culture with a donor's uninfected cells which have been stimulated with phitoemoglutinine (PHA) for 3 days. These co-cultures are controlled about every 3 days for 28 days or more to control either the syncitia formation and p24 presence or the reverse transcriptase (RT) in culture supernatant (2). The presence either of syncitia (a) or the p24 antigen (b) or the RT (c) is considered a virus replication evidence (2). According to Gallo et other authors even the finding of virus-like particles in culture must be considered a sufficient criterion.
a) The appearance of sincytia.
Sincytia appearance is observed only in considered infected cultures, but these are present even in “uninfected” cell lines and are similar to the ones used for HIV (25). Then such morphological feature could be a characteristic either of the same cell lines, or of the result of the culture conditions or both these factors (14).
b) The p24 antigen.
Its specificity lack has already been mentioned. The objections to use it like a test on the patient's serum are efficacious even for its use in cultures. In a study where this “virus isolation” method was employed ( the determination of the p24 in cultures with unfractioned entire blood), positive results were obtained in 49 of 60 “presumably uninfected but serologically undetermined individuals”(82%) and in 5 of 5 seronegative blood donors (26).
c) The reverse transcriptase (RT).
In all the HIV research, the ability of copying template-primer A(n)dT15 - when it is incubated with the supernatant or material coming from infected material co-cultures with the AIDS virus which separates at a 1.16 gm/ml level (sucrose density gradient) - is considered the proof of a reverse-transcriptase activity of HIV. In many cases this activity is considered synonymous of “HIV isolation” . However: also the template-primer itself is copied when it is incubated with material which separates in 1.16 band from leukemic cell cultures (27) and uninfected spermatozoa(29); b) the template-primer A(n)dT15 can be transcripted not only by RT, but also by other DNA cell polymerases. All the DNA cell polymerases, alfa, beta and gamma are able of copying the A(n)dT15 (29). In fact. in 1975, an International Conference on DNA which included Baltimore and Gallo (30) on eukariotic polymerases defined the DNA polymerase gamma, “a normal cell component” (31) which activity can be increased by many factors including the PHA stimulation (32), an enzyme which “copies the A(n)dT15 with high performance, but does not copy well the DNA” (33). From what over explained we may deduce that it is wrong to affirm that such an enzyme activity is peculiar of retroviruses, and less that this is an unequivocal index of the presence of HIV. Particles and proteins which separate at 1.16 mg/ml band level could reflect fully unviral material, most of the same material is certainly cellular and coming from cultures stimulated with PHA (as we shall see better later), since transcriptase activity could derive from it.
d) Objects with a retrovirus appearance.
Gallo considered the finding of retrovirus-like particles in culture like the proof of the HIV isolation (at that time HTLV-III) (34). However Gallo used H9 (HUT78) cell line to isolate HTLV-IIIB, and this cell line releases retrovirus-like particles even when it is not infected with HIV” (35). Weiss obtained his “isolate CBL-1 from the leukaemic cell line CEM, a cell line which hosted retroviruses even when they were not infected with “HIV”(36). In 1970, these particles were frequently observed in leukaemic tissues (37), in embryonic tissue cultures (38, 39) and in most if not in all human placenta (40). Mature and *extrobended* particles of the C pattern appear in linphoma cells which are metabolically damaged but are not infected with HIV (41). “Retrovirus particles” antigenically similar to HIV were found in extracts from Sjorgen syndrome patient's salivary glands(42). And more important thing, in the only study of electron microscopy(43), both in vivo and in vitro where suitable controls were used and where an extensive assay in cieco of controls and test material was made, “virus particles indistinguishable from HIV were found” in a variety of reactive linphoadenopathies which are not HIV-associated, so that the authors concluded: “The presence of such particles does not indicate, for itself, an HIV infection”.
Fig. 4 - Can these animals be considered quasi species of human being?
Their genetic difference is comparable to that presnt among various "HIV" genotypesConclusion
It is inevitable to agree that neither syncitia presence, nor p24 finding, nor reverse transcriptase activity, nor virus-like particle presence (well, each element which settles that an HIV culture is positive) are “a virus replication evidence, though just this is the significance given to it.
Direct identification of the nucleic acids
For direct identification of the nucleic acids one intends the Southern Blot, PCR techniques, the quantitative amplification techniques (RT PCR, bDNA, NASBA). The identification is based on the identification and amplification of little nucleotide sequences attributed to HIV, not of the entire viral genome. This research is made not on cultures, but direclty on patient's tissues (including blood).
a) Southern blot technique (technique which allows to separate and highlight nucleic acids of the searched kind).
In 1984 Gallo carried out the first study. Using the Southern blot hybridization technique (capable theoretically of identifying only one infected on ten cells), AIDS patient's tissues were examined, but weak bands were found only in a minority of them (44).
b) The PCR (polymerase chain reaction).
The PCR sensibility is valued 92-100% (45) and is able to detect one “infected” cell on 100.000 (2). However, “for its high sensibility, it is greatly liable to false positivity either for even minimum contaminations or other common laboratory mistakes” (2). Retrovirus-like sequences can be found in human genome in every cell (46). Already in 1991, Imagawa and Detels pointed out a potential fallacy of the PCR. “The determination of a part of the genome- they affirmed- does not mean to detect an entire virus” (47).
The problem of the false positives.
Many researchers challenge the PCR reability for the great number of false positive that this test should produce(48). The PCR did not prove reproducible and false positives and false negatives were observed in all the 7 reference laboratories involved in an important French study (the concordance with serology varied from 40% to 100%). Moreover, the number of positive results was not significally different among high and low risk seronegatives (49). There are many other confirmations to these remarks:
1) A validity study in evaluating the HIV PCR performance and in identifying free-cell viral DNA showed “a disturbing high rate of unspecific positivity“ employing commonly used primers (SK38, both for the p24 and the gag gene). Really, similar positivity rates were found either for antibody negative samples than for antibody negative samples(18% against 26%) (50).
2) The PCR (qualitative DNA-PCR) made on uninfected infants appeared positive on many occasions (51).
3) In a PCR validity study coordinated by the OMS Work Group on the PCR, 54% of involved laboratories had problems with false positive results; 9.3% of uninfected samples were reported as positive(52).
4) The authors of a multicenter study of quality evaluation stated “this study showed that false positive results, in spite of rigorous procedure algorithms, happen among uninfected individuals with such a frequency to remain a serious problem”(53). The discover of a considerable number of “false positives” gives evidence of a remarkable PCR specificity lack.
c) Quantitative techniques.
The “quantitative” techniques” should be able to calculate, in an approximate way, the number of virions per milliliter of plasma using opportune modifications of some amplification techniques (either substrate amplification, like RT-PCR and NASBA, or signal amplification, like bDNA.The substrate consists in “preserved regions” of a virus-presumed gene. The calculated sensibility is: for the RNA RT-PCR of 100 HIV RNA copies per milliliter of plasma(2); for NASBA of 1,000 molecules per milliliter (54); for bDNA (branched DNA) of 10,000 molecules per milliliter (2). Up to 1994 HIV was considered to be present in small quantities in asymptomatic and AIDS subject's tissues (1 lymphocyte on 1,000-100,000). In 1995 two studies (55, 56) seemed, on the contrary, to show that HIV replicated with much activity since from the first phases of the infection. The over-mentioned techniques in appearance allowed huge virus quantities, which in a first time had escaped from observation, to be detected.
However if a massive infection were present in infected individuals, as some of the most known experts claim, the Southern blot hybridization would have been more than sufficient to detect it. Don't forget that “either virus DNA scarcity or lack in a considerable patient's part was a surprising result”(57). A John Maddox' significant Freudian slip confirms suspects: according to Maddox “both Ho than Wei and their colleagues were able to come to their alarming conclusions only after a ten years research of the HIV as they teamed up with mathematicians and because they were able to use new techniques to detect the low levels of virus involved “(58)!
Logical incompatibilities of results are macroscopic and evident.
A) In fact there is a lack of correlation between p24 and gene amplification techiniques. (We have already mentioned the fact that the p24 is considered to be “ the direct proof of HIV identification”).
A) No relation was found between the p24 level and the RT-PCR (59). The determination of the viral charge was highly sensible when it was compared with the p24 measurement (more than 95% of patients had detectable virus charge while only 40% had detectable p24), but in positive samples antigen and viral charge level had only a borderline significance (60).
B) No relation was observed between viral charge quantified either with the NASBA or the Amplicor and the concentration with the p24 antigen. No relation was observed between the infectious dose, the viral virl load found either with the NASBA or the Amplicor(RT-PCR) (61).
C) There is a relation lack between RT-PCR and other assays. In a study on vaccinated subjects, a group of four serum samples resulted positive with RT PCR during a 11 months period, including a result of 104-105 copies/mL, and an year later a fifth positive result in a vaccinated subject, but not infected” (62). For this reason, the authors advanced “doubts concerning specificity of HIV RNA measurement as a golden standard for the screening and the infection confirmation”.
D) There is finally a lack of correlation between “virus” and “viral load”.
1) According to Piatak, the high “virus” level in plasma was 999 per 1,000 “unculturable” (63).
2) Ho et al. showed that 10,000 plasma “virions” counted with the branched PCR correspond with less than one “infectious” virus per ml (64).
3) In a more recent study by Ho (65) one may notice that 0 (zero!) “cultured viruses” corresponded to a load of 500,000 units! According to the reported data, to patient 105 before the treatment (with Ritonavir) 643,000 “virions”/ml were detected, where the number was calculated with the branched DNA assay, more than 1,000 TCID-50/ml corresponded to it (50% tissue culture infectious doses). After a two day therapy and in following days the “cultivable virus” was 0(zero). In the meantime, at 2, 3 and 4 days “free virions” (that is the so-called “viral charge”) remained over 500,000. Till 7 day “virions” were again over 100,000. A virion is defined as a complete viral particle constituted with the viral nucleic acid, capsid and pericapsid (when present). The possible objection for which a defective virion is “seen” by branched DNA assay, but is not detected by culture samples, does not resolve the problem of non-correspondence with the other tests and the impossibility of seeing these particles at the microscope directly from patient's tissues, without culture transfers. If it were so, Gallo would not need for applying to frauds (67, 68), he would not call for three year tireless researches in order to find something which had the appearance of a virus. He would have come to it immediately with other researchers.
Mark Craddock, professor at the School of Mathematics and Statistics, The University of Sidney, Australia commented: “What is this viraemia of billions of RNA particles that can only be seen with an undocumented branch-PCR or PCR but not with a functional infectivity test?”. (68)
VIRAL GENOME
As already stated, genomic amplification techniques concern small segments, not surely the complete “viral” genome and the results which are obtained do not give the assurance to have the true “HIV”, besides they are frankly discordant. At this point it's legitimate to ask what is really and how it's made the original viral genome. The reserch group from Perth (69) notes that if an unique AIDS retrovirus existed, then less than 1% genomic differences should be the rule, not the exception. For instance, the type 3 Sabin poliovirus vaccine differed from its neurovirulent progenitor at only 10 nucleotide positions after 53 in vitro and 21 in vivo passages in monkey tissues. In 1977, H1N1 influenza A virus reappeared in the human population after 27 years of dormancy with sequences mainly identical to those of the 1950s virus”. Although Eigen's quasispecies model has been used to describe the genome of RNA viruses, even 1% sequence differences in these genomes are considered to represent “extreme variability”. “Many selective forces may stabilize virus populations. These stabilizing factors may include the need for conservation of protein structure and function, RNA secondary structure, glycosylation sites, and phosphorylation sites”. Let us see what is the picture offered by HIV research. Not long afterwards it was discovered that “If you were to test two HIVpositive people at random and analyse the genetic material of their strains, they would differ, on average, by about 13 percent” (70). Besides what is considered to be the genotype of HIV comes from sequence analysis of subgenomes that HIV genotype consignments are derived from sequence analysis of subgenomes measuring 2% to 30% of the total (71). The data is that such “genomes” vary between 3-40% (72,73). “If 30% of the HIV genome varies as much as 40%, how much does 100% of the HIV genome vary? This is the legitimate question of Eleopulos and colleagues (69).
Among researchers there is disagreement also about the number of HIV genes. In 1988 they said HIV genome had eight genes (74), in 1990 ten (75). In 1996 Montagnier referred that HIV had eight genes (76), and according to Barré-Sinoussi, HIV has nine genes (77) Not even the number of nucleotides of HIV genome seems to be constant (69)
HIV quasi-species
Because great genomic differences were found - even the same person can harbor more than 106-108 genetically distinct variants (78) - to try to find a justification for this phenomenon, the concept of quasi-species has been introduced. Prior to the 1990s, the HIV sequences were classified as African and USA/European with sequence differences of 20-30 percent between these two groups. (79). In the 1990s, HIV researchers started to divide the “HIV genome” into subtypes A, B, C, D, E, etc. The basis for this classification system is: “(a) subtypes are approximately equidistant from one another in env; (b) the env phylogenetic tree is for the most part congruent with gag phylogenetic trees; (c) two or more samples are required to define a sequence subtype”. However, “Subtype naming problems have arisen. A small but not insignificant number of viral sequences are hybrid, clustering with one sequence subtype in gag and another sequence subtype in env, for example;... Naming becomes problematic when highly divergent forms of a given subtype arises: such forms are sometimes designated A', B', F', etc”. It is increasingly necessary to have sequence data from both gag and env coding sequences when a new form or subtype is being claimed” (80). By the middle of 1996 “at least ten” (A-J) prevalent major (M) and a low prevalence, O, HIV-1 genotypes were described and new genotypes are still reported (81,82).
Is it possible then to describe the “HIV DNA” even if it has variation of 10% , not to mention 20 or 30 or 40% as is the case, as a ...”population of closely related genomes, referred to as a quasispecies” (69)? The extreme variability of HIV genome make us ask what is the sense to refer to HIV as a clear distinct entity. The genomic difference among human being of different race is about 1 per 1.000, between man and orang-utan about 2%, but to find a 30% difference is necessary to compare non the less man and mouse, man and ass, man and elephant! Can one consider mouse, ass and elephant as quasi-species of man?.
Fig. 5 - Electronic microscopy of a cell surrounded by a small particles.
Without any proof these are considered viral particles (HIV), while
they can simply be artefacts of staining or cellular particles.Particle detection Retrovirus are defined as particles of about 100-120nM of diameter with a core which includes a proteic coat and a ribonucleoprotein complex. Retroviruses are classified in three subfamilies: Spumavirinae, Lentivirinae and Oncovirinae. Retroviruses belonging to the last subfamily are divided in type A, B, C, and D particles. To which of these should belong HIV, is not very clear. According Gallo in 1984, it was a type C particle, but in 1985 he admitted it could be also a type D (83). Montagnier and coll. initially reported it was a type C particle (84), then a type D (85), and then a lentivirus (86), that is to say a different subfamily. According to Munn and others AIDS virus shows characteristics of type C, of type D and of lentivirus (87). Fauci and others described HIV in monocyte/macrophages cultures as retroviral particles with characteristics of type A (88). HIV identity seems to be so vague, so “shot”, that it adds further reasons of perplexity. Photographs Several researchers in the AIDS field have shown HIV photographs, but those that can be seen are only virus-like viral particles indistinguishable from normal cellular microvescicles. These particles, in contrast with viruses, are very unstable when removed from their contest, and it's difficult to isolate them and photograph them in a condition of a true isolation. On the contrary, viruses are stable because they have to leave again the cells and host organisms. The use of centrifugation techniques doesn't constitute a problem to separate viruses from various contaminants and so get their isolation - then photograph them, to put in evidence their proteins and their genetic constituents in a direct way. True viruses are so stable that it's easy to photograph directly them as tridimensional particles by an electronic scanning microscope, without the need to first fix them chemically. On the contrary, the microvescicles are so unstable that can be photographed only by a transmission electronic microscope which requires that they be previously fixed chemically in ultra-thin sections. What has been shown to the world as HIV micro graphs are ultra thin sections which include particles indistinguishable from the cellular ones (89) (pict 6-8).
The evidence of HIV (non-)existence A noteworthy confirmation to the argued critics of the researchers from Perth came in march 1997: two groups, one French/German (90) and one from the American NCI (91), have published the photographs of density gradients in which pure HIV virus had to be present (pict. 9). These data should have been available by the first isolation of the virus (1983-84), but nobody showed them before. The most likely reason is the lack of correspondence between what they found and what they expected.
In the Franco/German study the pictures are from the 1.16 gm/ml band. It is impossible to tell from which density the pictures in the American study are taken but presumably it's the same 1.16 density for retroviral particles. Eleopulos and colleagues note that “. The authors of both papers concede that the particles which are present in the banded material and which are said to be HIV represent only a very small fraction of the total material. Gelderblom et al. state that the material contains “an excess of [cellular] vesicles with a size range 50-500nm, as opposed to a minor population of virus particles... cellular vesicles appear... to be a major contaminant of HIV preparations enriched by sucrose gradient centrifugation”. “For the small number of particles deemed to be “HIV” no evidence is given that they are even a retrovirus-like particle. Indeed, to the contrary: (a) the particles do not appear to have surface spikes (knobs)... In other papers published by many researchers including Gelderblom and his associates such projections are noted to be absent (93, 94); (b) the particles referred to as “HIV” are not spherical and have diameters exceeding 100-120 nM. In the EM in Gluschankof et al. (90) there are arrows pointing to five “HIV” particles devoid of surface projections whose dimensions are 121 X 145; 121 X 169; 121 X 145, 121 X 145 and 133 X 145 nM respectively.
In Bess et al. (91) there are a total of six “HIV particles” also devoid of surface projections whose dimensions are 160 X 240; 200 X 240; 280 X 280; 208 X 250; 167 X 250 and 250 X 292 and nM respectively. “Actually, the authors maintain these are HIV particles, but without any proof. In the material of that density band there ought to be many viral particles, on the contrary they point only some, among many impurities.
They bear only the vaguest resemblance to retroviral particles”. “The point is that any genuine retroviral particle contains a fixed amount of RNA and protein. No more and no less. This means that if we consider diameters of particles pointed in the two studies, they are 1.14 times to 1.96 times larger than the estimated maximum for a normal HIV particle. Translating this in volumes, and comparing them to a particle with a diameter of 120nM, the Franco/German particles have 50% more volume than a retroviral particle and the US particles have 750% more volume”, that is to say incompatible with the same definition of a retrovirus! Even if the authors do not admit it, the data of the two cited papers contribute to show that where should be present pure HIV, of HIV there is no trace.
CONCLUSION
None of the tests examined seems to be sufficiently sure to detect an HIV infection. Paradoxically not even the “direct virus detection” is able to do answer to the same question. As a matter of facts there is an incompatible discordance among the results obtained with different methods. These discordances are by themselves logical incompatibilities that invalidate them. Besides, the existence of an entity that one can reasonably name “HIV virus” is not proved, as far as its proteins, its genome, its physical particle are concerned. The true meaning of the tests is better understood if its origin is examined. To show the existence of a new virus, both Montagnier group in 1983 and the group of Gallo in 1984 separated supernatant of cultures they considered infected in density gradients. They erroneously considered that the material that banded at 1,16 mg/ml was constituted by “pure HIV particles”. The proteins which were present in this band and were reactive with AIDS patients sera (but not only with their sera) were considered HIV proteins. In the same manner, a particular fraction of RNA which they found in the same band was considered the genome of the new virus. By then, some of these proteins are used to get the reagent for some kind of tests or as immunogens to produce in laboratory animals the antibodies used for other kind of tests ( similar procedure was followed to obtain RNA segments).
At this point the explanation of Gallo experiments in 1984 appears clearer: he used a mixture of proteins (believed to be viral but in fact cellular) to immunize the rabbits and obtain a serum which obviously reacted with the same proteins that were in the culture from which the initial mixture came (95).
In other words Gallo believed to show an hypothesis (the existence of the virus) with other hypothesis shown to be false (that is to say that inverse transcription activity, the presence of virus-like particles, the antibodies obtained in the way just mentioned were all virus specific).
In this way were born the tests which to-day are still used and perfectioned to put so engaging a diagnosis. An important prove of the correctness of this interpretation of the facts stays in the results that they obtained on mice and monkeys in 1991 (96).
In those studies, with the scope to evaluate a candidate vaccine, some laboratory animals were injected with cellular components from cultures which were “not infected by HIV”: the results were similar to those obtained with “the real vaccine” (i.e. with virus from “infected cultures”).
No significant difference was found, so that the comment - perfectly suited and still up to date of John Maddox - was: “AIDS research turned upside down”.
That is to say that it wasn't necessary at all to hypothesize the presence of a virus to obtain those phenomena.
Dr. Fabio Franchi MD, Specialista in Igiene, Medicina Preventiva e in Malattie Infettive, Trieste
References:
1) Maddox J. Finding wood among the trees. Nature 1988; 335:11.
2) Metcalf JA, Davej Jr. RT and Lane C. Serologic and Virologic tests. In De Vita VT Jr, Hellman S, Rosenberg SA, Curran J, Essex M and Fauci AS. AIDS: Biology, Diagnosis, Treatment and Prevention, 4th edition. Lippincott-Raven Publishers, 1997; pp. 177-195.
3) Ministero della Sanità. Dipartimento per la valutazione dei medicinali e la farmacovigilanza. Linee guida per la terapia antiretrovirale dell'infezione da HIV. Bollettino d'informazione sui farmaci 1996; 6-7:3-13.
4) Commissione Nazionale per la Lotta all'AIDS. Gli screening per gli anticorpi anti-HIV. The Practitioner (ed. it.) 1987; 3:101-120.
5) Voevodin A. HIV screening in Russia (letter). Lancet 1992; 339:1548.
6) Lundberg GD. Serological Diagnosis of Human Immunodeficiency Virus Infection by Western Blot Testing. JAMA 1988; 260:674-679.
7) Ministero della Sanità. AIDS - la diagnosi, il test , il counselling. 1992.
8) Meyer KB and Pauker SG. Screening for HIV: Can we afford the false positive rate? NEJM 1987; 317: 238-241.
9) Zolla-Pazner S. et al. Reinterpretation of HIV Western Blot Patterns. N Eng J Med 1989; 320:1280-1.
10) Midthum K, Garrison L, et al. Frequency of indeterminate Western Blot tests in healthy adults at low risk for HIV infection. J Infect Dis 1990; 162:1379-82.
11) Mac Kenzie WR et al. Multiple False -positive Serologic tests for HIV, HTLV-1, and Hepatitis C Following Influenza Vaccination. Jama 1991; 268:1015-17.
12) Kozhemiakin LA, Bondarenko IG. Genomic instability and AIDS. Biochimiia 1992; 57:1417-1426.
13) Strandstrom HV, Higgins JR, Mossie K, et al. Studies with canine sera that contain antibodies which recognize human immunodeficiency virus structural proteins.
Cancer Res 1990; 50:5628s-5630s.
14) Eleopulos EP, Turner VF and Papadimitriou JM. Is a positive Western Blot Proof of HIV Infection? Bio/Technology 1993; 11:696-707.
15) Vogt Advances in virus research. 1965; 11:293-383.
16) Bourinbaiar AS. HIV and GAG. Nature 1991; 349:111. (100 pg p24 equivalent to 1.000.000 HIV particles).
17) Bialy H. Where is the virus? and where is the press? BIO/Technology 1988; 6:121.
18) Bourinbaiar AS. HIV and GAG. Nature 1991; 349:111.
19) Paul DA, Falk LA, Kessler A et al. Correlation of serum HIV antigen and antibody with clinical status in HIV-infected patients. J Med Virol 1987; 22(4): 357-63.
20) Todak G, Klein E, Lange M et al. A clinical appraisal of the p24 Antigen test, p. 326. In: Vol. I, Abstracts VII International Conference on AIDS, Florence, 1991.
21) Delord B, Ottmann M, Schrive MH et al. HIV-1 expression in 25 infected patients: A comparison of RNA PCR, p24 EIA in Plasma and in situ Hybridization in mononuclear cells, p. 113. In: Vol. I, Abstracts VII International Conference on AIDS, Florence, 1991.
22) Genesca J, Jett BW, Epstein JS & Bloggs. What do Western Blot indeterminate patterns for Human Immunodeficiency Virus mean in EIA-negative blood donors? Lancet 1989; ii:1023-1025.
23) Mortimer P, Codd A, Connolly J, et al. Towards error free HIV diagnosis: notes on laboratory practice. Pub Health Lab Service Microbiol Digest 1992; 9:61-64.
24) Valore Predittivo Positivo = VP/(VP+FP); VP = veri positivi, FP = falsi positivi.
25) Poiesz B, Ruscetti FW, Mier JW, et al. T-cell lines established from human T-lymphocytic neoplasias by direct response to T-cell growth factor. Proc Natl Acad Sci 1980; 77:6815-6819.
26) Schüpbach J, Jendis JB, Bron C et al. 1992. False-positive HIV-1 virus cultures using whole blood. AIDS 6: 1545-1546.
27) Gallo RC, Sarin PS and Wu AM. On the nature of the Nucleic Acids and RNA Dependent DNA Polymerase from RNA Tumor Viruses and Human Cells, p. 13-34. In: Possible Episomes in Eukaryotes. LG Silvestri (Ed.). North-Holland Publishing Company, Amsterdam, 1973.
28) Whitkin SS, Higgins PJ and Bendich A. Inhibition of reverse transcriptase and human sperm DNA polymerase by anti-sperm antibodies. Clin Exp Immunol 1978; 33:244-251.
29) Sarngadharan MG, Robert-Guroff M, Gallo RC. DNA polymerases of normal and neoplastic mammalian cells. Biochim Biophysica Acta 1978; 516:419-487.
30) Weissbach A, Baltimore D, Bollum F, et al. Nomenclature of eukaryotic DNA polymerases. Science 1975; 190:401-402.
31) Robert-Guroff M, Schrecker AW, Brinkman BJ, et al. DNA polymerase gamma of human lymphoblasts. Biochem 1977; 16:2866-2873.
32) Lewis BJ, Abrell JW, Smith RG, et al. Human DNA polymerase III (R-DNA): Distinction from DNA polymerase I and reverse transcriptase. Science 1974; 183:867-869.
33) Weissbach A, Baltimore D, Bollum F, et al. Nomenclature of eukaryotic DNA polymerases. Science 1975; 190:40-402.
34) Gallo RC, Salahuddin SZ, Popovic M, et al. Frequent Detection and Isolation of Cytopathic Retroviruses (HTLV-III) from Patients with AIDS and at Risk for AIDS. Science 1984; 224:500-502.
35) Dourmashkin RR, O'Toole CM, Bucher D, Oxford JS. The presence of budding virus-like particles in human Iymphoid cells used for HIV cultivation. Vllth International Conference on AIDS. Florence: 1991:122.
36) Minassian A, Merges M, Garrity R, et al. Induction of a SMRV-like retrovirus from a human T-cell line after treatment with the mutagen ethyl-methyl-sulfonate. J Acquir Immun Defic Syndr 1993; 6 (No 6):738.
37) Gallo RC, Wong-Staal F, Reitz M et al. 1976. Some Evidence For Infectious Type-C Virus in Humans, p. 385-407.
In: Animal Virology. D Baltimore, AS Huang, CF Fox (Eds.). Academic Press Inc., New York.
38) Panem S, Prochownik EV, Reale FR et al. Isolation of Type C Virions from a Normal Human Fibroblast Strain. Science 1975; 189:297-299.
39) Panem S, Prochownik EV, Knish WM and Kirsten WH. Cell Generation and Type-C Virus Expression in the Human Embryonic Cell Strain HEL-12. J Gen Virol 1977; 35:487-495.
40) Panem S. C Type Virus Expression in the Placenta. Curr Top Pathol 1979; 66:175-189.
41) Brennan JK, Lichtman MA, Chamberlain, JK and Leblond P. Isolation of Variant Lymphoma Cells with Reduced Growth Requirements for Extracellular Calcium and Magnesium and Enhanced Oncogenicity. Blood 1976; 47:447-459.
42) Garry RF, Fermin CD, Hart DJ et al. Detection of a Human Intracisternal A-Type Retroviral Particle Antigenically Related to HIV. Science 1990; 250:1127-1129.
43) O'Hara CJ, Groopmen JE and Federman M. The Ultrastructural and Immunohistochemical Demonstration of Viral Particles in Lymph Nodes from Human Immunodeficiency Virus-Related Lymphadenopathy Syndromes. Hum Pathol 1988; 19:545.
44) Shaw GM, Hahn BH, Suresh KA et al. Molecular Characterization of Human T-Cell Leukemia (Lymphotropic) Virus Type III in the Acquired Immune Deficiency Syndrome.
Science 1984; 226: 1165-1171.
45) Schleupner CJ. Detection of HIV-1 infection. In Mandell/Douglas/Bennet. Principles and Practice of Infectious Diseases. Churchill-Livingstone, IV Edition, 1995: 1253-67.
46) Lanka S. HIV: reality or artifact? Continuum May 1995; 4-9.
47) Imagawa D, Detels R. HIV-1 seronegativehomosexual man. N Engl J Med1991; 325:1250-1.
48) Sloand EM et al. HIV testing - State of the Art (Review). JAMA 1991; 266:2861-6.
49) Defer C et al. Multicentre quality control of polymerase chain reaction for detection of HIV DNA. AIDS 1992; 6:659-63.
50) Busch MP, Henrard DR, Hewlett IK et al. The Transfusion Safety Study Group. Poor sensitivity, specificity, and reproducibility of detection of HIV-1 DNA in serum by polymerase chain reaction. J Acquir Immune Defic Syndr 1992; 5(9): 872-7.
51) Paul MO, Tetali S, Lesser ML et al. Laboratory diagnosis of infection status in infants perinatally exposed to human immunodeficiency virus type 1. J Infect Dis 1996, Jan; 173(1): 68-76.
52) Bootman JS, KItchin PA. An international collaborative study to assess a set of reference reagents for HIV-1 PCR. J Vir Meth 1992; 5:872.
53) Sheppard HW, Ascher MS, Busch MP et al. A multicenter proficiency trial of gene amplification (PCR) for the detection of HIV-1. J Acquir Immune Defic Syndr. 1991; 4(3): 277-83.
54) Schuurman R, Descamps D, Weverling GJ, et al. Multicenter comparison of three commercial methods for quantification of human immunodeficiency virus type 1 RNA in plasma. J Clin Microbiol.1996 Dec; 34(12): 3016-22.
55) Ho DD, Neumann AU, Perelson AS, et al. Rapid turnover of plasma virions and CD4 lymphocytes in HIV-1 infection. Nature 1995; 373:123-126.
56) Wei X, Ghosh SK, Taylor M, et al. Viral dynamics in human immunodeficiency virus type 1 infection. Nature 1995; 373:117-122.
57) Simmonds P, Balfe P, Peutherer JF, et al. Human immunodeficiency virus-infected individuals contain provirus in small numbers of peripheral mononuclear cells and at low copy numbers. J Virol 1990; 64:864-872.
58) Maddox J. (review of Hodgkinson book AIDS, the failure of contemporary science) The Guardian Newspaper 1996 July 5th; page 16.
59) Coste J, Montes B, Reynes J, et al. Comparative evaluation of three assays for the quantitation of human immunodeficiency virus type 1 RNA in plasma. J Med Virol. 1996 Dec; 50(4): 293-302.
60) Vandamme AM, Schmit JC, Van Dooren S, et al. Quantification of HIV-1 RNA in plasma: comparable results with the NASBA HIV-1 RNA QT and the AMPLICOR HIV monitor test. J Acquir Immune Defic Syndr Hum Retrovirol. 1996 Oct 1; 13(2): 127-39.
61) Gobbers E, Fransen K, Oosterlaken I, et al. Reactivity and amplification efficiency of the NASBA HIV-1 RNA amplification system with regard to different HIV-1 subtypes. J Virol Methods. 1997 Jul; 66(2): 293-301.
62) Schwartz DH, Laeyendecker OB, Arango Jaramillo S, et al. Extensive evaluation of a seronegative participant in an HIV-1 vaccine trial as a result of false-positive PCR. Lancet 1997 Jul 26; 350(9073): 256-9.
63) Piatak MJ, Saag MS, Yang LC, et al. High levels of HIV-1 in plasma during all stages of infection determined by competitive PCR. Science. 1993; 259:1749-1754.
64) Cao Y, Qin L, Zhang L, Safrit J, Ho DD. Virologic and immunologic characterization of long-term survivors of human immunodeficiency virus type 1 infection. N Engl J Med. 1995; 332:201-208.
65) Perelson AS et al. ( Ho D included). HIV-1 dynamics in vivo: virion clearance rate, infected cell life-span, and viral generation time . Science 1996; 271:1582-6).
66) Palca J. Draft of Gallo Report Sees the Light of Day. Science 1991; 253:1347-1348.
67) Cohen J. HHS. Gallo Guilty of Misconduct. Science 1993; 259:168-170.
68) Buianouckas FR. HIV an illusion. Nature 1995; 375:197.
69) Eleni Papadopulos Eleopulos, Valendar F Turner, John M Papadimitriou, David Causer. The isolation of HIV - has it really been achieved? Continuum Sept/Oct 1996; 4(3) S:1-24.
70) Brown P. The strains of the HIV war. New Scientist 1991; (25th May):14-15.
71) Salimen MO, Carr JK, Burke DS, et al. Genotyping of HIV-1. The human retroviruses and AIDS Compendium on Line: Web site: http://hiv-web.lanl.gov. USA: US Government, 1996: 30-34.
72) Zhu T, Wang N, Carr A, et al. Evidence or coinfection of multiple strains of human immuodeficiency virus type 1 B an acute seroconvertor. J Virol 1995; 69:1324-1327.
73) Kozal MJ, Shah N, Shen N, et al. Extensive polymorphisms observed in HIV-1 clade B protease gene using high-density oligonucleotide arrays. Nat Med 1996; 2:753-759.
74) Gallo R, Wong-Staal F, Montagnier L, Haseltine WA, Yoshida M. HIV/HTLV gene nomenclature. Nature 1988; 333:504.
75) Lazo PA, Tsichlis PN. Biology and pathogenesis of retroviruses. Semin Oncol 1990; 17:269-294.
76) Cunningham AL, Dwyer DE, Mills J, et al. Structure and function of HIV. Med J Aust 1996; 164:161-173.
77) Barré-Sinoussi F. HIV as the cause of AIDS. Lancet 1996; 348:31-35.
78) Wain-Hobson S. Virological mayhem. Nature 1995; 373:102.
79) Blomberg J, Lawoko A, Pipkorn R, et al. A survey of synthetic HIV-1 peptides with natural and chimeric sequences for differential reactivity with Zimbabwean, Tanzanian and Swedish HIV-1-positive sera. AIDS 1993; 7:759-767.
80) Myers G. Nucleic acids alignments and sequences. The human retroviruses and AIDS Compendium on Line: Web site: http://hiv-web.lanl.gov. USA: US Government, 1995: I-1-I-2.
81) Barré-Sinoussi F. HIV as the cause of AIDS. Lancet 1996; 348:31-35.
82) Salimen MO, Carr JK, Burke DS, et al. Genotyping of HIV-1. The human retroviruses and AIDS Compendium on Line: Web site: http://hiv-web.lanl.gov. USA: US Government, 1996: 30-34.
83) Gallo RC, Shaw GM, Markham PD. The etiology of AIDS. In: De Vita V, Hellman S, Rosenberg SA, ed. AIDS etiology, diagnosis, treatment, and prevention. New York: J. B. Lippincott Company, 1985: 31-51.
84) Barré-Sinoussi F, Chermann JC, Rey F. Isolation of a T-Lymphotrophic Retrovirus from a patient at Risk for Acquired Immune Deficiency Syndrome (AIDS). Science 1983; 220:868-871.
85) Klatzmann D, Barr-Sinoussi F, Nugeyre MT. Selective Tropism of Lymphadenopathy Associated Virus (LAV) for Helper-Inducer T Lymphocytes. Science 1984; 225:59-63.
86) Montagnier L. Lymphadenopathy-Associated Virus: From Molecular Biology to Pathogenicity.
Annals of Internal Medicine 1985; 103:689-693.
87) Munn RJ, Preston MA, Yamamoto JK, Gardner MB. Ultrastructural comparison of the retroviruses associated with human and simian acquired immunodeficiency syndromes. Laboratory Investigation 1985; 53:194-199.
88) Orenstein JM, Meltzer MS, Phipps T, Gendelman HE. Cytoplasmic assembly and accumulation of human immunodeficiency virus types 1 and 2 in recombinant human colony-stimulating factor-1-treated human monocytes: an ultrastructural study. Journal of Virology 1988; 62:2578-2586.
89) Lanka Stephan. No viral identification: non cloning as proof of isolation! Continuum Feb/March 1997; 4(5):31-33.
90) Gluschankof P, Mondor I, Gelderblom HR, Sattentau QJ. Cell membrane vesicles are a major contaminant of gradient-enriched human immunodeficiency virus type-1 preparations. Virol. 1997; 230:125-133.
91) Bess JW, Gorelick RJ, Bosche WJ, et al. Microvesicles are a source of contaminating cellular proteins found in purified HIV-1 preparations. Virol 1997; 230:134-144.
92) Eleni Papadopulos-Eleopulos. A critique of the evidence for the isolation of HIV (A summary of the views of Papadopulos et al.) Rethinking aids web site (http://www.virusmyth.com/aids) epsummary.htm 1997.
93) Gelderblom HR, Ozel M, Hausmann EHS, Winkel T, Pauli G, Koch MA. Fine Structure of Human Immunodeficiency Virus (HIV), Immunolocalization of Structural Proteins and Virus-Cell Relation. Micron Microscopica 1988;19:41-60.
94) Hockley DJ, Wood RD, Jacobs JP. Electron Microscopy of Human Immunodeficiency Virus. J Gen Virol 1988; 69:2455-2469.
95) Popovic M, Sarngadharan MG, Read E, et al. Detection, Isolation, and Continuous Production of Cytopathic Retroviruses (HTLV-III) from Patients with AIDS and Pre-AIDS. Science 1984; 224:497-500.
96) Maddox J. Aids research turned upside down. Nature 1991; 353:2.